Hey! Check out our New Interactive Periodic Table with rotating Bohr models—it's a game-changer!
Take a look!🙂
For future use, bookmark this Periodic table
or visit "PeriodicTableGuide.com"
Periodic table Groups: The vertical columns in the periodic table are known as groups of periodic table. There are total 18 groups (vertical columns) on the periodic table.

So you have seen the above periodic table labeled with group names (from 1-18). Right?
Now let’s see 1-18 individual groups of Periodic table along with their group names.
Let’s dive straight into it !!
Groups in Periodic Table (With Group Names)
There are total 18 different groups in Periodic table.
- Group 1: Alkali metals group (hydrogen not included)
- Group 2: Alkaline earth metals group
- Group 3-12: Transition and Inner transition metals group
- Group 13: Boron group
- Group 14: Carbon group
- Group 15: Nitrogen group
- Group 16: Oxygen group
- Group 17: Halogen group
- Group 18: Noble gases group
Let me explain each of these groups in short.
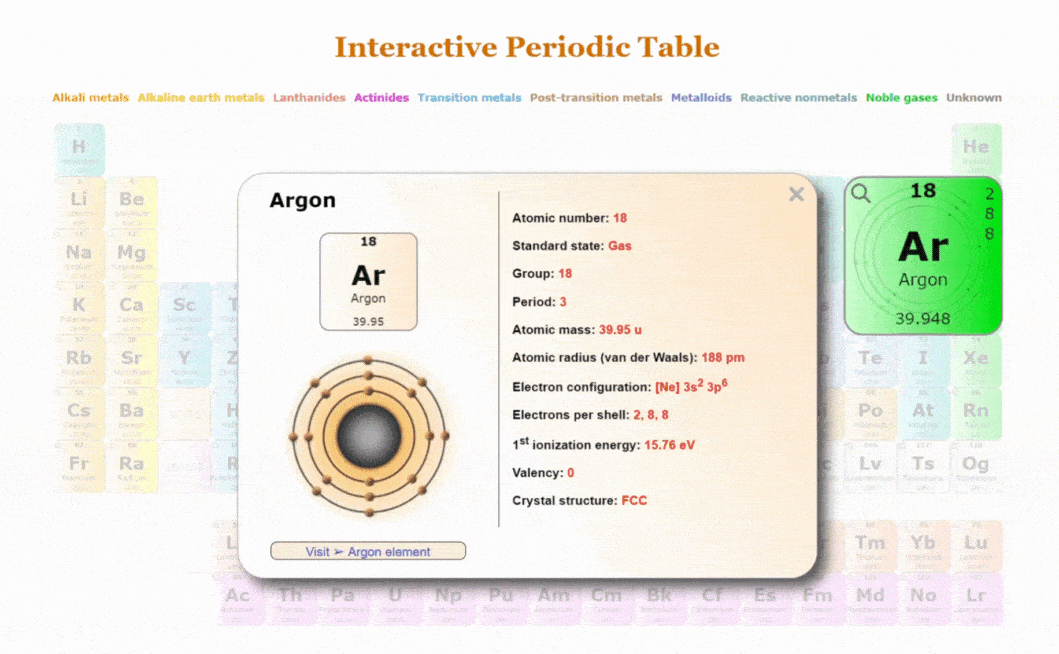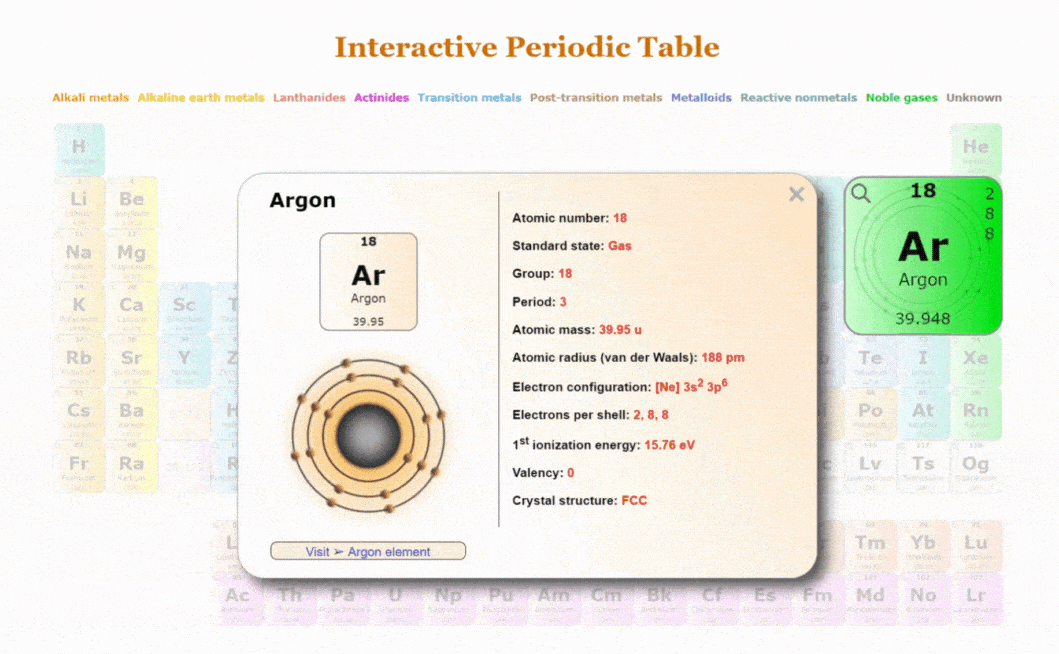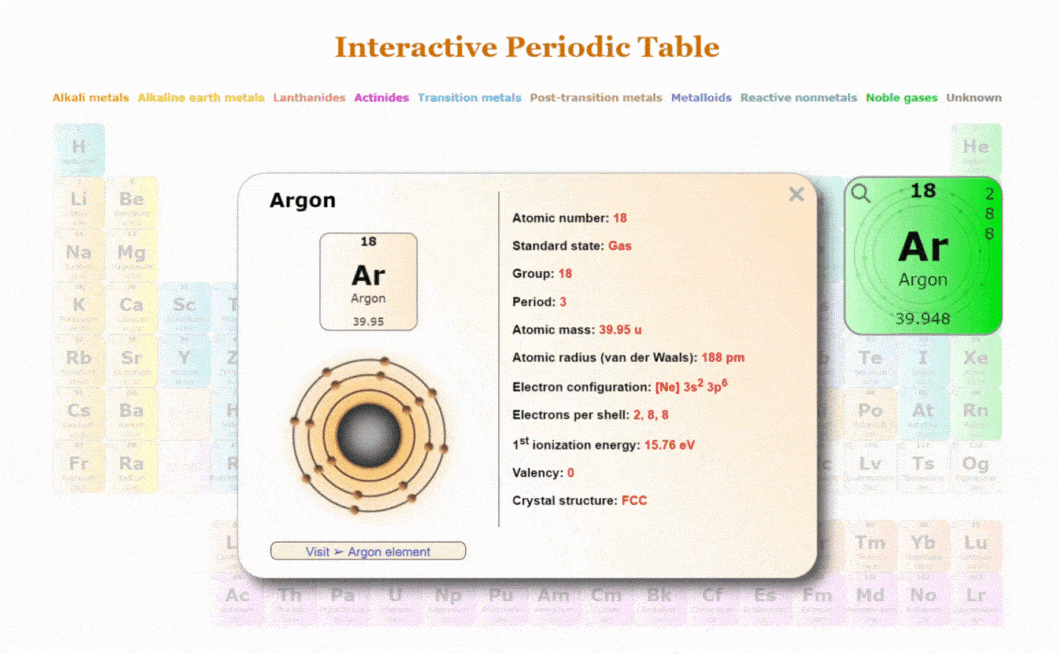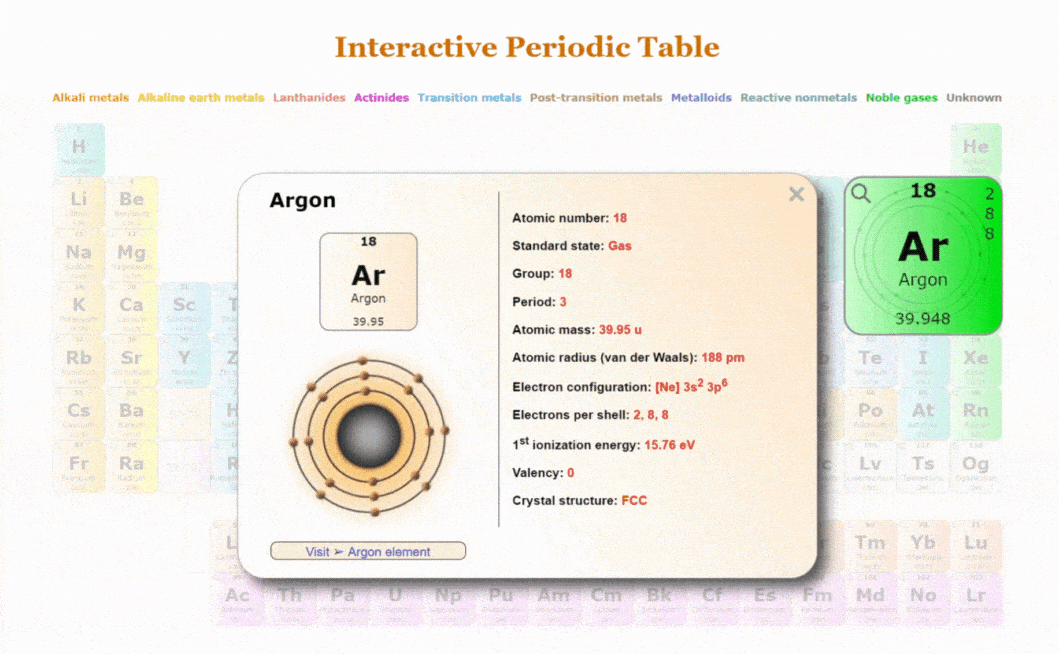
Explore our New Interactive Periodic Table with Rotating Bohr Models
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Visit ➢ Periodic table
For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Group 1: Alkali metals group

Alkali metals group is the very first group (group 1) on the periodic table.
The elements included in the Alkali metals group are;
- Lithium (Li)
- Sodium (Na)
- Potassium (K)
- Rubidium (Rb)
- Cesium (Cs)
- Francium (Fr)
For detailed information on Alkali metals, read the Ultimate guide on Alkali metals of periodic table.
Also see: Why Alkali metals are so reactive to water?
Group 2: Alkaline earth metals group

Alkaline earth metals are the group 2 elements on the periodic table.
The elements included in Alkaline earth metals group are;
- Beryllium (Be)
- Magnesium (Mg)
- Calcium (Ca)
- Strontium (Sr)
- Barium (Ba)
- Radium (Ra)
For detailed information on alkaline earth metals, read the main article on Alkaline earth metals on periodic table.
Also see: Why are group 2 elements called alkaline earth metals?
Group 3-12: Transition and Inner transition metals group

The elements lying from group 3 to 12 on the periodic table are named as Transition metals and Inner transition metals.
The elements in the two bottom rows of the periodic table are also included in these groups.
They are placed in the two separate rows at the bottom because they show few different properties.
Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3.
But as these elements have few different properties, they are grouped as separate elements known as inner transition elements.
For detailed information on transition and inner transition metals, read the main articles on Transition metals on periodic table and Inner transition metals on periodic table.
Group 13: Boron group

Boron group is the group 13 on the periodic table.
The elements included in boron group are;
- Boron (B)
- Aluminum (Al)
- Gallium (Ga)
- Indium (In)
- Thallium (Tl)
- Nihonium (Nh)
Also see: Complete information on Boron element.
Group 14: Carbon group

Carbon group is the group 14 on the periodic table.
The elements included in carbon group are;
- Carbon (C)
- Silicon (Si)
- Germanium (Ge)
- Tin (Sn)
- Lead (Pb)
- Flerovium (Fl)
Also see: Complete information on Carbon element.
Group 15: Nitrogen group

Nitrogen group is the group 15 on the periodic table.
The elements included in Nitrogen group are;
- Nitrogen (N)
- Phosphorus (P)
- Arsenic (As)
- Antimony (Sb)
- Bismuth (Bi)
- Moscovium (Mc)
Also see: Complete information on Nitrogen element.
Group 16: Oxygen group

Oxygen group is the group 16 on the periodic table.
The elements included in Oxygen group are;
- Oxygen (O)
- Sulfur (S)
- Selenium (Se)
- Tellurium (Te)
- Polonium (Po)
- Livermorium (Lv)
Group 17: Halogen group

Halogen group is the group 17 on the periodic table.
The elements included in halogen group are;
- Fluorine (F)
- Chlorine (Cl)
- Bromine (Br)
- Iodine (I)
- Astatine (At)
- Tennessine (Ts)
For detailed information on halogens, read the main article on Halogen elements of Periodic table.
Group 18: Noble gases group

Noble gases group is the last group (group 18) on the periodic table.
The elements included in Noble gases group are;
- Helium (He)
- Neon (Ne)
- Argon (Ar)
- Krypton (Kr)
- Xenon (Xe)
- Radon (Rn)
- Oganesson (Og)
For detailed information on noble gases, read the main article on Noble gases of periodic table.
Final words
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups.
The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
For example;
Example of group 1
All the elements of group 1 are highly reactive to water. They are soft and can be cut easily with a kitchen knife.
Also all the elements of group 1 have one valence electron.
Example of group 18
All the elements of group 18 are chemically inert (that means they do not easily react with other elements).
And all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell).
In this way, the elements of the same group show similar chemical properties and they also have the same number of valence electrons.
Explore our NewInteractive Periodic Table (with Rotating Bohr Models and More)
Details about this Periodic table:
- Access detailed infoon all elements: atomic mass, electron configurations, charges, and more.
- Viewrotating Bohr modelsfor all 118 elements.
- Get a freeHD imageof the Periodic Table.
Visit ➢ Periodic Table
Download HD image
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”